Molecular Formula: C₄H₁₀BF₃O
Molecular Weight: 141.94
CAS Number: 109-63-7
Abbreviations and Synonyms: Boron Trifluoride Diethyl Etherate, Boron Trifluoride Ethyl Etherate, Boron Trifluoride Ether Complex
A bright yellow liquid with a boiling point of 126°C and a bf3 etherate density of 1.15 g/cm³. It is soluble in most organic solvents, including benzene, toluene, various chlorinated methanes, ethers, methanol, 1,4-dioxane, and tetrahydrofuran (THF). It is commonly used in dichloromethane. Boron trifluoride diethyl etherate is also known as bf3 etherate.
Boron trifluoride etherate is commercially available from domestic and international reagent suppliers and is generally not synthesized in laboratories.
It oxidizes slowly in air, turning black; therefore, it is best purified by distillation before use. Boron trifluoride diethyl etherate msds provides detailed safety information.
Highly moisture-sensitive, it reacts violently with water to release toxic fluorinated gases.
It must be used in an anhydrous system and handled in a fume hood.
BF₃-OEt₂ is a commonly used and easily handled substitute for BF₃ gas. As a Lewis acid, it catalyzes:
The ring-opening and rearrangement of epoxides.
The esterification of carboxylic acids.
The cleavage of trityl ethers.
As a Lewis acid, BF₃-OEt₂ enhances the reactivity of nucleophilic reagents in various addition reactions, significantly increasing the activity of TMSCN, allylsilanes, organocopper lithium reagents, and enol silyl ethers. Boron trifluoride diethyl etherate uses in this context are extensive.
For instance, under mild conditions, allylsilanes can undergo SN2 substitution with Morita–Baylis–Hillman adducts, yielding 1,5-diene derivatives with high regio- and stereoselectivity (Figure 1) [1].
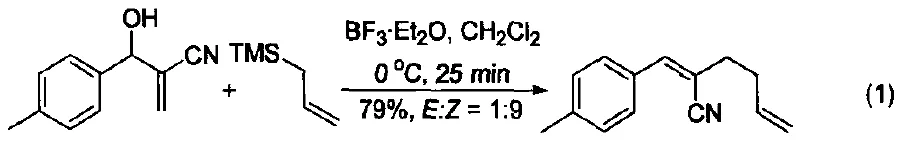
BF₃-OEt₂ is frequently used to catalyze the Diels-Alder cycloaddition between furan and methyl acrylate, producing adducts with excellent endo-selectivity [2].
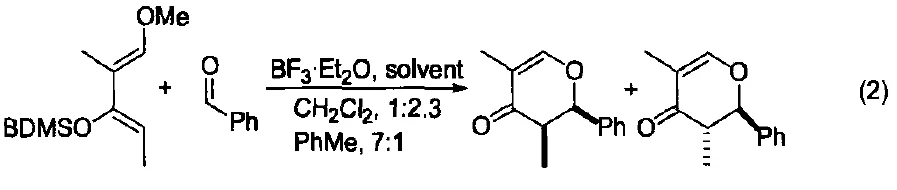
It also catalyzes heteroatom cycloaddition reactions between aldehydes and enol silyl ethers, yielding pyrone derivatives.
By selecting an appropriate solvent, the stereoselectivity of these reactions can be effectively controlled (Figure 2) [3].
BF₃-OEt₂ is widely used in the formation and removal of protective groups:
In aprotic solvents, BF₃-MeOH gently removes trityl ether protection with excellent functional group compatibility [4].
BF₃-OEt₂, combined with iodide ions, facilitates mild cleavage of alkyl ethers and acetal protection. Unlike other acidic boron reagents, it does not cleave aromatic ethers under these conditions [5,6].
In chloroform or dichloromethane, BF₃-OEt₂ efficiently removes TBDMS protection [7].
The ethanol solution of BF₃-OEt₂ is extensively used for the esterification of various alkyl and aryl carboxylic acids. Even relatively unstable substrates can be esterified under these mild conditions [8].
It also promotes the condensation of carboxylic acids with amines to form amides, with the reaction rate being enhanced by adding a base or using azeotropic water removal [9].
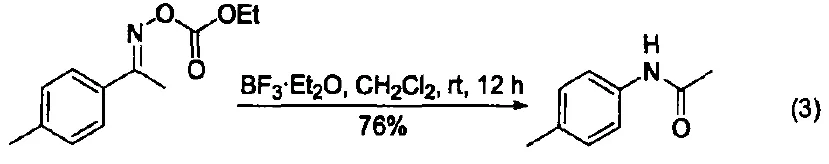
BF₃-OEt₂ catalyzes the Beckmann rearrangement of various ketoxime carbonates at room temperature, yielding amides with good yields (Figure 3) [10].
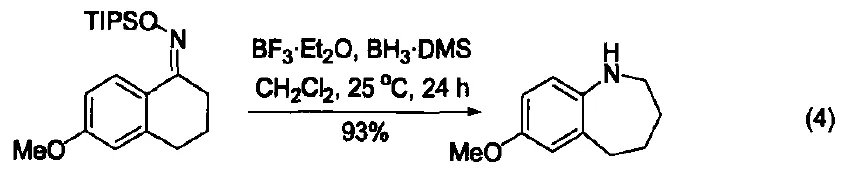
In cooperation with borane, BF₃-OEt₂ also catalyzes the Beckmann rearrangement of silyl-protected ketoximes. Due to the reductive properties of borane, the reaction directly yields various aniline derivatives (Figure 4) [11].
BF₃-OEt₂ is also involved in Pinacol coupling reactions:
Under the co-catalysis of trichloromethylsilane and BF₃-OEt₂, imines and aldehydes undergo Zn-Cu alloy-mediated cross-Pinacol coupling, forming vicinal amino alcohols, though with low stereoselectivity (Figure 5) [12].
This system also catalyzes the cross-coupling of two imines, with significantly improved stereoselectivity (Figure 6) [13].
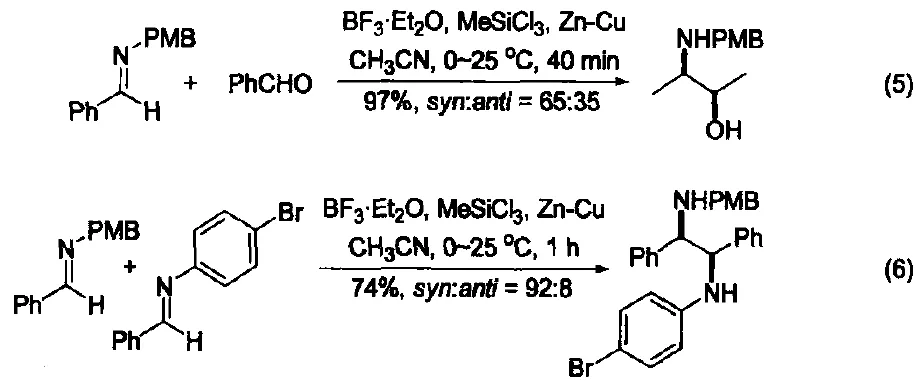
BF₃-OEt₂ is commonly used in various reduction reactions:
Unlike metal halides, BF₃-OEt₂ catalyzes the hydrosilylation of aldehydes and ketones at room temperature, leading to the formation of ethers and borate esters for aliphatic aldehydes and ketones.
For aromatic ketones, such as acetophenone or benzophenone, it facilitates their reduction to ethylbenzene and diphenylmethane [14].
When used with NaBH₄, BF₃-OEt₂ efficiently reduces carboxylic acid derivatives, including acyl chlorides, esters, amides, nitriles, and carboxylic acids [15].
Under BF₃-OEt₂ catalysis, imines can be reduced by tributyltin hydride [16].
References
1. Yadav, J. S.; Reddy, B. S.; Mandal, S. S.; Singh, A. P.; Basak, A. K. Synthesis 2008,1943.
2. Kotsuki, H.; Asao, K.; Ohnishi, H. Bull. Chem. Soc. Jpn. 1984,57,3339.
3. Danishefsky, S.; Chao, K.-H.; Schulte, G. J. Org. Chem. 1985,50,4650.
4. Mandal, A. K.; Soni, N. R.; Ratnam, K. R. Synthesis 1985, 274.
5. Mandal, A. K.; Shrotri, P. Y.; Ghogare, A. D. Synthesis 1986,221.
6. Pelter, A.; Ward, R. S.; Venkateswarlu, R.; Kamakshi, C. Tetrahedron 1992, 48,7209.
7. Kelly, D. R.; Roberts, S. M.; Newton, R. F. Synth. Commun. 1979,9, 295,
8. (a)Hinton, H. D.; Nieuwland, J. A. J. Am. Chem. Soc. 1932, 54, 2017. (b) Sowa, F. J.; Nieuwland, J.A. J. Am. Chem. Soc. 1936,58,271. (c) Hallas, G. J. Chem. Soc. 1965, 5770.
9. Tani, J.; Oine, T.; Inoue, I. Synthesis 1975,714.
10. Anilkumar, R-;Chandrasekhar, S. Tetrahedron Lett. 2000, 5427.
11. Ortiz-Marciales, M.; Rivera, L. D.; Jesu’s, M. D.; Espinosa, S.; Benjamin, J. A.; Casanova, O. E.;Figueroa, I. G.; Rodriguez, S.; Correa, W.7. Org. Chem. 2005, 70,10132.
12. Shimizu, M.; Iwata, A.; Makino, H. Synlett 2002,1538.
13. Shimizu, M.; Suzuki, I.; Makino, H. Synlett 2003,1635.
14. Kotsuki, H.; Asao, K.; Ohnishi, H. Bull Chem. Soc. Jpn. 1984, 57,3339.
15. Cho, S.-D.; Park, Y.-D.; Kim, J.-J.; Falck, J. R.; Yoonm, Y.-J. Bull Kor. Chem. Soc. 2004,25,407.
16. Ueda, M.; Miyabe, H.; Namba, M.; Nakabayashi, T.; Naito, T. Tetrahedron Lett. 2002, 43,4371.
This is the last one.